1250
Views & Citations250
Likes & Shares
EMBRYONIC AND ADULT STEM CELLS
Stem cells have entered the center stage of biomedical research for more than two decades and are key to the study of their biology and differentiation, of early human development as well as their therapeutic potential.
Stem cells are somatic cells which are naturally generated by meiosis from diploid cells and conjugation of a haploid egg cell (22 autosomes and 1 sex chromosome X) with a haploid sperm cell (22 autosomes and 1 sex chromosome X or Y) in the process of fertilization. The resulting diploid chromosomal set is made up of 44 autosomes and 2 sex chromosomes XX (female karyotype) or XY (male karyotype). The fertilized egg cell divides to yield a 2-, 4-, 8-, 16-, 32- … -cell stage to form - through the morula, blastomere and blastocyst (day 6-14) stage- within 84 days the embryo and subsequently the fetus with delivery of the newborn on day 273-281 (Figure 1A).
Stem cells are somatic cells with different proliferation and differentiation potential. Embryonic stem cells (ES) are toti- or pluripotent and are derived prenatally from blastocysts. Adult stem cells (AS) are multipotent and can be isolated postnatally from peripheral blood and many tissues or organs and can be used for different therapeutic applications after in vitro differentiation into the desired cell types and tissues (‚tissue engineering’). With the development from embryo to fetus the stem cells lose their totipotency (blastomere stage: ability to form all cell types, including oocytes and sperm cells) and become pluripotent (blastocyst stage: ability to form all cell types of the embryo, fetus and newborn/ adult) and can differentiate into several cell types (multipotency). Differentiated cells, such a skin, liver or myocardial cells, are unipotent and can, under physiological conditions, reproduce only the same cell type.
Milestones of stem cell research were the characterization of the first ES with hematopoietic potential in mice (mES) in 1981 [1] for which Sir Martin Evans (UK) was awarded the Nobel Prize for Physiology or Medicine in 2007 together M. Capecchi and O. Smithies (both USA).
CLONING BY NUCLEAR TRANSFER
A further milestone of stem cell research was the cloning of the sheep ‚Dolly’ by nuclear transfer from an adult sheep skin cell into an enucleated egg cell [2,3] and the generation of human embryonic stem cells (hES) [4] or of primate embryonic stem cells (pES) [5]. From blastocysts of monkey embryos two ES lines were isolated with all characteristics of ES (DNA identical to skin cell ‚donor’ DNA, mitochondrial DNA of the egg cell, differentiation in vitro into myocardial and nerve cells and in vivo into teratomas which are derived from ektoderm, entoderm and mesoderm. These data demonstrate that it is possible to reprogram adult somatic cells into pluripotent stem cells by nuclear transfer and to clone primates for therapeutic purposes, a strategy that in principle is also applicable in humans [6]. While this is prohibited by the 2016 Guidelines for Stem Cell Research and Clinical Translation issued by the International Society for Stem Cell Research (ISSCR) this strategy has, for example, been successfully applied to clone a Banteng cow, a relative of the domesticated cattle and an endangered species. Obviously, this strategy could be extended to other endangered species while cloning of pets is at least debatable.
INDUCED PLURIPOTENT STEM CELLS
In 2006 Takahashi and Yamanaka from the University of Kyoto/ Japan reported another milestone in stem cell research (Figure 1B): using unipotent fibroblasts from adult mice they could reprogram them in vitro to so-called induced pluripotent stem cells (mIPS) by retroviral transduction and integration of 4 genes into the cellular genome (mOct4, mSox2, mKlf4 and mc-Myc) which were almost identical to mES [7]. They extended these findings with human fibroblasts [8] which were independently reported by Yu et al. at the University of Wisconsin-Madison, Madison, USA [9]. The genes’ code for transcription factors involved in the control of the ‚selfrenewal’ of mES and hES, respectively [10]. Subsequently, the generation of IPS has been independently reproduced and optimized [11-13].
The hIPS were practically indistinguishable from hES with respect to morphology, proliferation kinetics, surface markers, the epigenetic make-up specific for pluripotent cells, and telomerase activity. Further, IPS differentiated into cells of ectoderm, entoderm and mesoderm as well as into teratomas. The Kyoto group could further show that IPS can also be generated by transduction of only 3 genes (hOct4, hSox2 and hKlf4, without mc-Myc gene) into adult human or mouse fibroblasts [14]. This strategy was somewhat less efficient than the transduction of 4 genes, but at the same time it was more specific with fewer tumors developing (0% versus 16% after 4-gene transduction).
For the generation of mIPS and hIPS by reprogamming of adult cells S. Yamanaka received the Nobel Prize for Physiology or Medicine in 2012 together with J. Gordon from the UK.
The discovery of Yamanaka and his team at the University of Kyoto [7,8] and of James A. Thomson and his team at the University of Wisconsin-Madison, Madison, USA [9] revolutionized stem cell research by making the generation of hES independent from human embryos. IPS have a tremendous potential for the molecular and biologic study of the pathogenesis of human diseases and their treatment, such as sickle cell anemia in a mouse model [15]. Further, IPS can be considered as autologous cells with the potential to serve as source for cell and organ transplantation (‚regenerative medicine’), theoretically without the risk of rejection. On the other hand, the retroviral or lentiviral transfer and chromosomal integration of genes conferring pluripotency to the IPS in principle carries a risk for chromosomal rearrangements, including a malignant transformation. Therefore, problems associated with using retroviruses and oncogenes for reprogramming need to be resolved before IPS cells are considered for use in humans.
FROM IPS TO BLASTOIDS: A NOVEL PLATFORM FOR THE STUDY OF EARLY HUMAN DEVELOPMENT
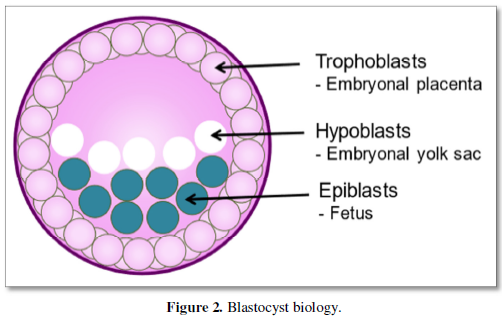
Recently, a novel milestone in the field of early human development was independently reported by a research team at the University of Texas Southwestern Medical Center, Dallas, TX, USA [16] and scientists at Monash University, Victoria, Australia, respectively [17]. By reprogramming human fibroblasts in vitro to IPS and applying a three-dimensional culture strategy, it was possible to generate blastocyst-like structures (Figure 1C), termed ‚human blastoids’ and ‚iBlastoids’, respectively [16,17]. The characterization of the blastocyst-like structures revealed an inner cell mass made up of epiblasts -that develop into the embryo/ fetus- and hypoblasts -that develop into the embryonal egg yolk sac- as well as trophoblasts -that will become part of the placenta- very similar to human blastocysts (Figure 2). They further very much resemble human blastocysts (Figure 1A), among others, in terms of morphology, size, cell number and allocation of different cell lineages. They recapitulate key morphogenetic events that occur during human peri-implantation development. Single-cell transcriptomic analyses confirmed the presence of epiblast-, entoderm- and trophoectoderm-like cells. In addition, iBlastoids give rise to pluripotent and trophoblast stem cells and allow to model several aspects of the early stage of implantation in vitro [17].
While stem-cell-derived blastocyst-like structures have initially been in generated in mice that allowed the analyses several pre- and peri-implantation developmental processes in vitro [18, 19] the novelty of the strategy lies in the extension to human blastoids [16, 17]. Human blastoids were shown to be an alternative to blastocysts for studying early human development. While several limitations still exist (variable derivation efficiency, low efficiency of generating early post-implantation structures and others), human blastoids provide a valuable and versatile model for basic and translational research as an alternative to human blastocysts which require fertilized human eggs (Figure 1A). The 2016 Guidelines for Stem Cell Research and Clinical Translation issued by the International Society for Stem Cell Research (ISSCR) do not permit research on cultures of human embryos or embryo-like structures beyond 14 days post-fertilization. The 2016 Guidelines for Stem Cell Research and Clinical Translation issued by the ISSCR are presently under revision to define the status of blastoids and their use.
SUMMARY AND PERSPECTIVES
Stem cells have entered the center stage of biomedical research for more than the two decades and are key to the study of their biology and differentiation, of early human development as well as their therapeutic potential. Milestones of stem cell research were the discovery of the embryonal stem cells (ES) with hematopoietic potential in mice (mES) [1], the cloning of animals by nuclear transfer [2,3] and the generation of human embryonic stem cells (hES) [4] or of primate embryonic stem cells (pES) by nuclear transfer from a skin cell into an egg cell from which the nucleus had been removed [5]. Another major milestone was the reprogramming of adult cells, such as keratinocytes derived from a skin biopsy, by viral transduction of genes to generate iPS [7-9], that have a tremendous potential in basic as well as translational sciences.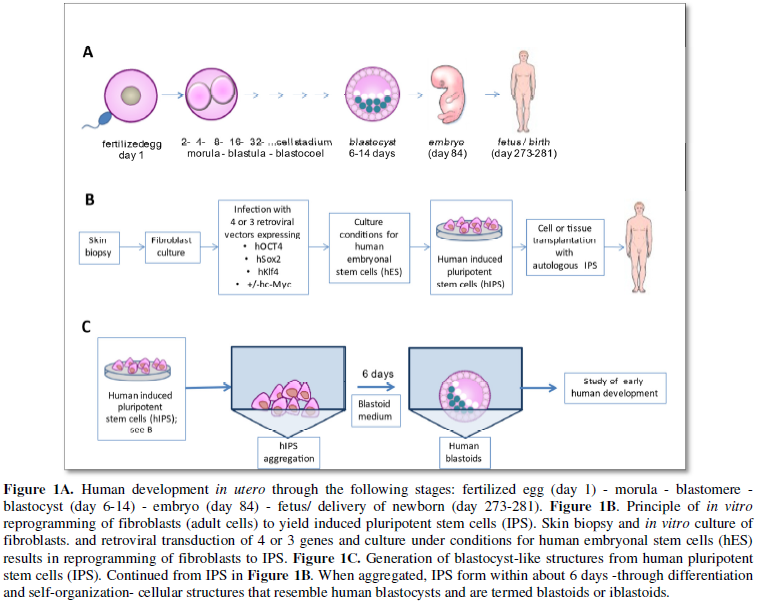
Blastoids are the most recent milestone in stem cell research documented by two independent seminal publications [16,17]. As lab-grown structures human blastoids mimic human embryo’s earliest stage of development [20]. They are in vitro models for embryo development and can be studied as an alternative to human embryos. Human blastoids provide a readily accessible and versatile alternative to blastcysts and are expected to give insights into different aspects of early human development, including early pregnancy loss, early developmental defects, effects of gene mutations and toxins during early embryogenesis, as well as the development of new therapies in the context of in vitro fertilization.
ACKNOWLEDGEMENTS
The excellent secretarial assistance of Mrs. Mariette Gutgsell and the valuable contribution of PD Dr. Tobias Boettler to the art work are gratefully acknowledged.
-
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154-156.
- Campbell KH, McWhir J, Ritchie WA, Wilmut I (1996) Sheep cloned by nuclear transfer from a cultured cell line. Nature 380: 64-66.
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385: 810-813.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282: 1145-1147.
- Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, et al. (2007) Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450: 497-502.
- Vogel G, Holden C (2007) Developmental biology. Field leaps forward with new stem cell advances. Science 318: 1224-1225.
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663-676.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861-872.
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917-1920.
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, et al. (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122: 947-956.
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, et al. (2007) Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1: 55-70.
- Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448: 313-317.
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, et al. (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448: 318-324.
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, et al. (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26: 101-106.
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, et al. (2007) Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318: 1920-1923.
- Yu L, Wei Y, Duan J, Schmitz DA, Sakurai M, et al. (2021) Blastocyst-like structures generated from human pluripotent stem cells. Nature 591: 620-626.
- Liu X, Tan JP, Schroder J, Aberkane A, Ouyang JF, et al. (2021) Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591: 627-632.
- Rivron N C, Frias-Aldeguer J, Vrij E J, Boisset J C, Korving J, et al. (2018) Blastocyst-like structures generated solely from stem cells. Nature 557: 106-111.
- Li R, Zhong C, Yu Y, Liu H, Sakurai M, et al. (2019) Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell 179: 687-702.
- Subbaraman N (2021) Lab-grown structures mimic human embryo's earliest stage yet. Nature 591: 510-511.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Diabetes (ISSN: 2644-3031)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Pathology and Toxicology Research



